This is the end... or not!
Sadly, this MSCA project ended on 31 January 2024. On the 5th of February I started as an assistant professor at Universidad Complutense de Madrid, teaching Geology to the students of the Chemistry degree. In addition to preparing the classes I had to prepare the final report for the EU. Now that the course is almost finished and I have finished the review of the comments made by the EU on my report I have time to update this website with the latest progress I have made on the project.
In January, we finally published a paper corresponding to the first work package I had proposed in the project, i.e. on the study of sulphate and carbonate minerals formation and, specifically, on bassanite formation. In this paper, which is available in open access, we have managed to synthesise bassanite at temperatures below 100C. And why is this breakthrough important?
Bassanite, CaSO4 ½H2O, is a sulphate widely used in construction, because when mixed with water it dissolves to recrystallise in the form of gypsum crystals, generating a resistant material that is present in our homes, as it is a fundamental component of plasterboard. Currently, the synthesis of bassanite is done by heating the gypsum at high temperatures (> 150 °C). Heating the gypsum to such high temperatures consumes a lot of energy and therefore also emits CO2.
In this work, we have used very saline solutions equilibrated with gypsum seeds (i.e. small gypsum crystals in suspension). These suspensions, kept under stirring and heated to 90 °C, yielded bassanite in a few minutes, which proved to be stable in suspension for a long time.
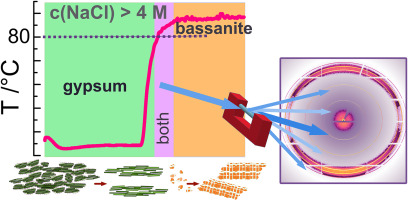
Graphical abstract of the paper. It can be seen a time vs. temperature graph showing how gypsum rapidly converts to bassanite as the temperature increases. (Source: Stawski et al., 2024)
One of the methods we have used to monitor the evolution of these reactions is in situ Raman, which is available at ISTerre. I have specialised in this method during my two-year contract in France. Thanks to in situ Raman, we were able to observe minute by minute, by the changes in the Raman signal, how the gypsum dissolved in the solution to give rise to the crystallisation of the bassanite.
To finish with this entry, which will not be the last, it only remains for me to mention that we are currently working on two more papers, one of them under review focused on the formation of gypsum and the other one focused on the carbonation of sulphate minerals to capture CO2 (which has been the research on which work packages 2 and 3 of the project have focused). When these papers are published, I will write new entries on the website. In addition, we have also published during this time 3 papers on foliar fertilisation, in which some of the chemical compounds that we have obtained as final product in the sulphate carbonation experiments have been used as fertilisers. These articles are also freely available in the publications section of this website.
I can only say goodbye until the next entry, which I hope to be able to publish soon (because it will mean that we have published one of the two remaining articles).
As the French would say, Au revoir!